Abstract

Umbilical cord blood transplantation (CBT) offers a readily available donor source with relatively limited GVHD even in the HLA-mismatched setting. However, due to the inherent naïveté of CB T-cells, virus-specific T-cell immune reconstitution is delayed which increases the risk for virus-related morbidity and mortality. We developed a novel manufacturing approach to engineer CB-derived multivirus specific T cells (CB-VSTs) specific for BK virus CMV, EBV and Adenovirus (Adv) for CBT recipients (CHEERS protocol, NCT03594981). This approach utilized autologous-CB derived dendritic cells (DCs), K562s genetically engineered to express costimulatory molecules, and PHA blasts (PHA-B) pulsed with overlapping Pepmixes spanning the entire length of the protein for the viruses of interest to stimulate T-cells in 3 weekly stimulations with harvest of the CB-VSTs on day 28.
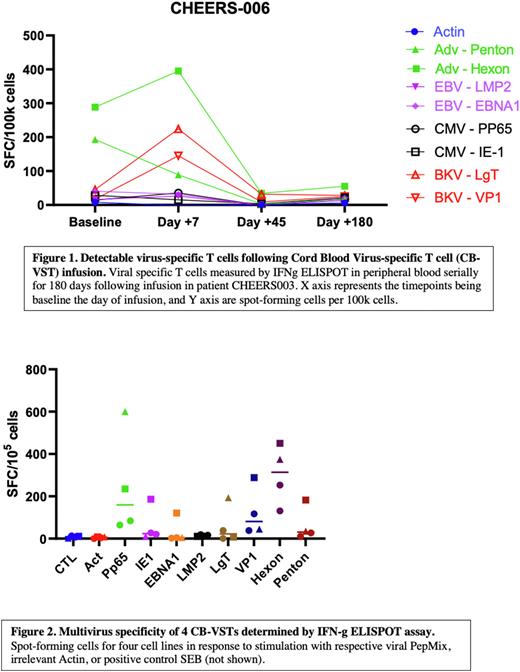
So far, one patient has received this quadrivalent CB-VST product. CHEERS006 is a 6-year-old male who received the CB-VST product 171 days post CBT. The resulting product was polyclonal and largely comprised of T cells (74.9%), with both CD4+ (49.5%) and CD8+ (21.9%) populations and a low proportion of CD56+ NK cells (1%). Intracellular cytokine staining (ICS) assay following restimulation showed consistent polyfunctionality, and IFNg ELISPOT assay showed that the expanded product was specific for the viral antigens of interest, with more than 30 spot-forming cells for Hexon and Penton (Adv), LMP2 (EBV) and Large-T (BKV). CHEERS-006 completed the 180-day safety monitoring period without severe adverse events attributable to the CB-VST product and remains well without recurrence of his primary hemoglobinopathy. Post-infusion the patient had a minor flare of skin (face) GVHD (grade 1/stage1) that responded to a 2-week steroid taper without recurrence. Prior to VST infusion, the patient had a history of adenoviremia reaching a peak of 21,559 copies/mL (60 days prior to VST infusion) and resulting in diarrhea, electrolyte derangements and malnutrition; treatment with Cidofovir was also limited because of nephrotoxicity. Post-infusion, he has had no adenoviremia and continues to remain negative for CMV, BKV and EBV on PCR surveillance. Virus-specific T cell responses post-infusion were also evaluated using IFNg ELISPOT in response to stimulation with viral antigens and showed expansion of VSTs early (within 7 days) post infusion (Figure 1).
With a lack of commercially available K562 cells, the manufacturing approach for CB-VSTs subsequently required modification. We hypothesized that similar results could be achieved solely using DCs and PHA-B as antigen presenting cells (APCs). This new approach was first tested with two cord blood samples and we observed a mean 10-fold expansion of CB-VSTs, demonstrating the feasibility of this method. We next sought to evaluate different cytokine combinations (including the existing GMP protocol) in order to better prime the virus-specific response, inhibit apoptosis and promote expansion of multivirus-specific T cells. A tool was developed to score 7 conditions based on fold expansion as well as specificity of VSTs via IFNg ELISPOT and ICS. Based on data from 4 CB-VST products we concluded that the use of IL-7, IL-12, IL-15 and IL-6 at initiation, IL-15 at STIM 2, and IL-2 at STIM 3 provided the optimal setting for growth and specificity of VSTs. At harvest, VST products expanded a mean of 10-fold, meeting the minimum for clinical dose. VSTs were polyclonal, comprised of mostly T cells (73.1%), with both CD4+ helper (33.4%) and CD8+ cytotoxic (64.7%) populations, and NK populations (0.8%). Products were polyfunctional and elicited robust multivirus specific T cell responses in vitro, secreting IFNg in response to most antigens when compared to the negative control actin (Figure 2).
In summary, we have developed a robust approach to manufacture VSTs targeting EBV, CMV, BKV and ADV from CB in the donor-specific setting to safely enhance anti-viral immunity and prevent viral infection after CB transplant.
Disclosures
Davila Saldana:SOBI: Consultancy. Shpall:Navan: Consultancy; axio: Consultancy; Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; adaptimmune: Consultancy; Bayer: Honoraria; Takeda: Patents & Royalties; Affimed: Other: License agreement. Hanley:Cellenkos: Consultancy, Other: Scientific Advisory Board; Cellevolve: Consultancy, Other: Scientific Advisory Board; Mana Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; MicroFluidx: Consultancy, Other: Scientific Advisory Board; Discovery Life Sciences: Consultancy, Other: Scientific Advisory Board. Bollard:Neogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Catamaran Bio: Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Scientific Co-Founder and SAB Member; Neximmune: Current equity holder in private company; Repertoire Immune Medicine: Current equity holder in private company; Cabalatte Bio: Membership on an entity's Board of Directors or advisory committees; Cellmedica: Patents & Royalties; SOBI: Other: DSMB Member; Mana Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties: VSTs/TAA-A; Roche: Consultancy; Pfizer: Consultancy; BMS: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal